Abstract
Introduction: Proteins such as Ferritin (Ft), Hemoglobin (Hb) and Transferrin Receptor (TfR1) are involved in tissue iron distribution while systemiciron metabolism is regulated by Hepcidin. Interleukin-6 (IL-6) release is induced in response to an inflammatory status and enhances Hepcidin expression, as consequence, dietary iron absorption and macrophage iron recycling are inhibited. Anemia of Chronic Disease (ACD) is a form of anemia associated with many different chronic disorders. It is characterized by high hepcidin and CRP or IL-6 expression and low serum iron transferrin concentration while ferritin can be normal or elevated. Oral iron delivery is especially attractive thanks to the ease of administration, but commonly used iron salts are poorly absorbed and ineffective to increase Hb concentration in particular in patients with ACD. We have developed an oral Iron formulation named Sucrosomial® Iron, preparation of ferric pyrophosphate, covered by a phospholipids plus sucrester matrix, with high bioavailability and tolerability, which promotes ferric iron absorption thanks to gastro-resistant and matrix composition properties and showed Hb concentration improvement not inferior to intravenous iron.
Objective: to showpre-clinical and clinical evidences of absorption, effectiveness and tolerability of Sucrosomial Iron using in vivo and in vitro models and in patients with ACD too.
Methods: To study the effects of oral supplementation on Iron metabolism and inflammatory status, Hb, Ft, TfR1, Socs3 and Hepcidin expression were analyzed. We have supplemented wild-type (wt) and iron deficient (ID) mice, maintained in iron-free diet for 8 weeks, with 1mg/kg/die of Sucrosomial® Iron or Iron Sulfate or Placebo by oral gavage, for 2 weeks. The role of Sucrosomial® Iron on the inflammatory response, was studied in LPS induced HepG2 hepatoma cells. Cells were treated with Sucrosomial® Iron and the levels of hepcidin were assessed 6, 18 and 24 hours after treatment. To show the efficacy and compliance of Sucrosomial® Iron a multi-centric randomized study on 300 patients with ACD were performed. Patients were supplemented with iron sulfate (65 mg o.i.d.), microencapsulated iron, micronized iron, Sucrosomial® Iron, heminic-chelated bisglycinated and iron chelated bisglycinated iron (30 mg t.i.d.). Median Hb value at the beginning was 8.2 g/dl. In group of patients with high CRP value median Hb value was 7.8 g/dl.
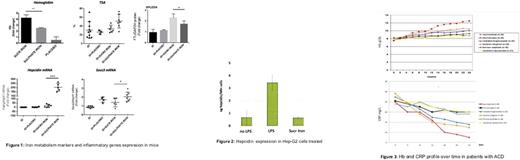
Results: In mice treated with Sucrosomial® Iron, Hb level mean increase was 4,9 g/dl while TSAT value is not significantly higher than animals treated with Sulfate Iron. Furthermore mice treated with Sucrosomial® Iron showed a preferential accumulation of iron in spleen. Hepcidin and Socs3 mRNA expression was not induced during Sucrosomial® Iron treatment while was up-regulated in mice treated with Iron Sulfate (Figure 1). In vitro experiments on HepG2 LPS-induced cells displayed that treatment with Sucrosomial Iron® is able to reduce hepcidin concentration at different time points (Figure 2).
Data from the clinical study showed that Sucrosomial® Iron is well tolerate and effective ACD patients. Only patients treated with Sucrosomial® Iron showed constant increase in Hb concentration over time. In all groups Hb level achieves a plateau phase after three months and ferritin level starts to increase. At three months higher levels of Hb are present in Sucrosomial® Iron (13.2 g/dl), heminic chelated bisglycinated iron (11.7 g/dl), chelated bisglycinated iron (11.3 g/dl) treated patients. In patients with high CRP level (>30 ng/ml) only those receiving Sucrosomial® Iron showed Hb increase from the tenth week and this continue up to the sixth month (Hb 12.5 g/dl) and seems to be correlated to a significantly decrease of CRP (5 mg/L) (Figure 3). Side effects are higher in ferrous sulfate (15/50) and sunactive treated groups (6/60).
Conclusions: In summary, results showed that Sucrosomial® Iron treatment could regulate iron homeostasis through an increase in Hb concentration and better tolerability during inflammatory status. These evidences may suggest that this new oral iron formulation behaves differently than other oral iron salts, perhaps due to different absorption pathways. Besides we have observed a decrease in CRP and Hepcidin concentration, these results should be further investigate.
Brilli: Pharmanutra S.p.A.: Consultancy. Pera: Pharmanutra S.p.A.: Employment. Tarantino: Pharmanutra S.p.A.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal